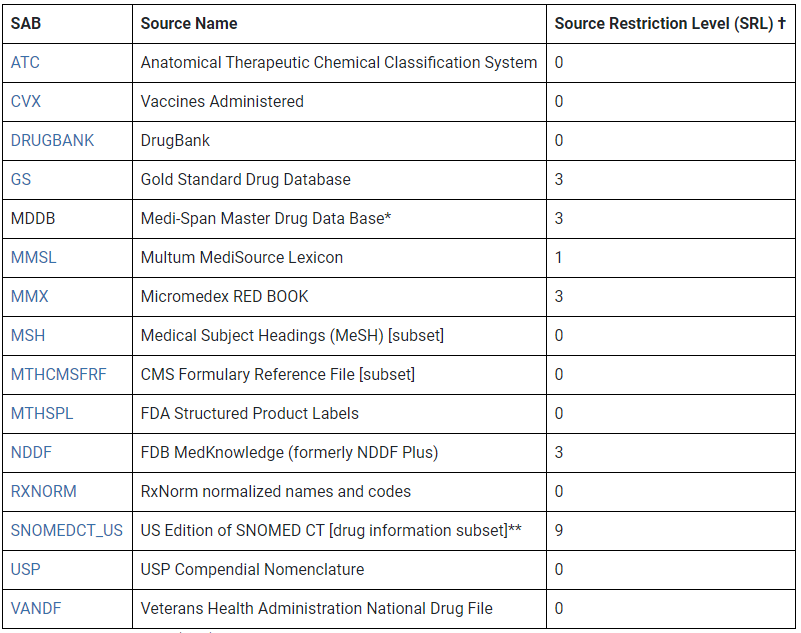
45 fda structured product labels
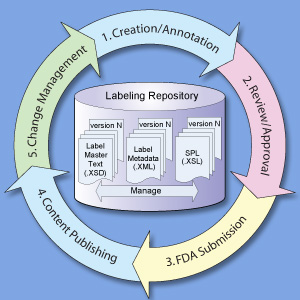
Structured Product Labeling Resources | FDA Aug 17, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. Lexis | Online Legal Research | LexisNexis The surprising truth about content … Fact: Lexis ® has the largest collection of case law, statutes and regulations.* Plus 40K+ news sources, 83B+ Public Records, 700M+ company profiles and documents, and an extensive list of exclusives across all content types.
August 23, 2021 Approval Letter - Comirnaty - Food and Drug ... Aug 23, 2021 · U.S. Food & Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 Our STN: BL 125742/0 . BLA . APPROVAL . BioNTech Manufacturing GmbH

Fda structured product labels
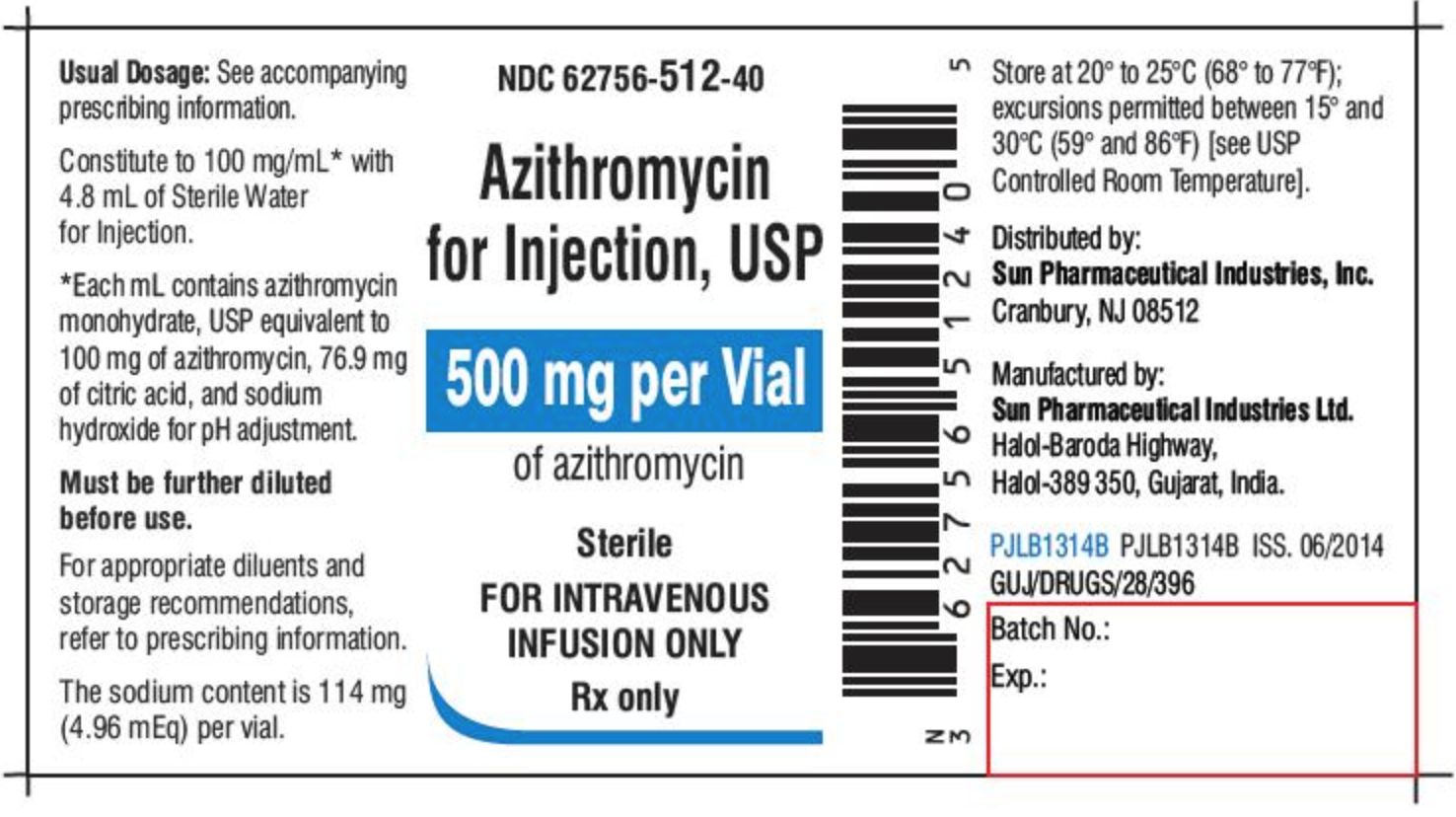
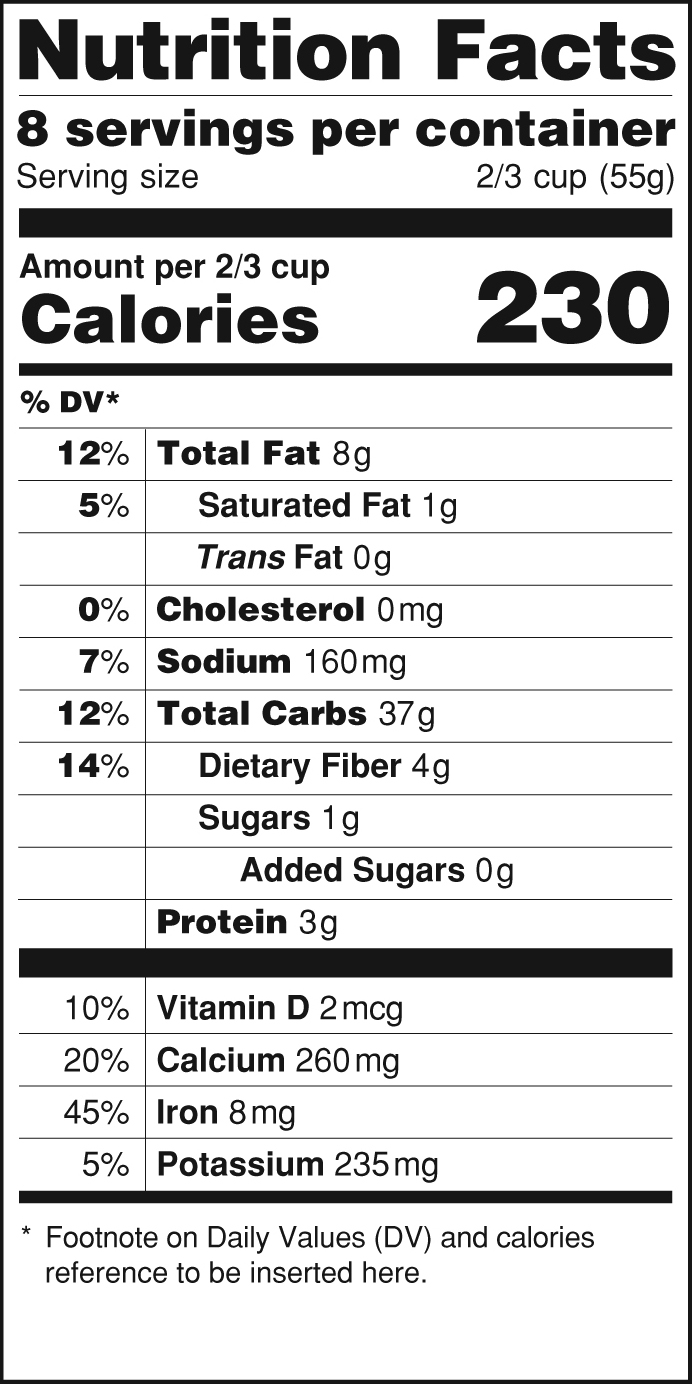
Federal Register :: National Bioengineered Food Disclosure ... Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302. IIS COVID-19 Vaccine Related Code | CDC The following vaccine NDCs and associated tradenames have been either submitted for FDA authorization (Pre-Authorization) or have been authorized or approved by the FDA under EUA or BLA License and may be included in FDA NDC files and Structured Product Labels (SPL). FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.)
Fda structured product labels. NSDE | FDA - U.S. Food and Drug Administration Mar 31, 2022 · With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for inclusion in the FDA Online Label Repository at labels.fda.gov. FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) IIS COVID-19 Vaccine Related Code | CDC The following vaccine NDCs and associated tradenames have been either submitted for FDA authorization (Pre-Authorization) or have been authorized or approved by the FDA under EUA or BLA License and may be included in FDA NDC files and Structured Product Labels (SPL). Federal Register :: National Bioengineered Food Disclosure ... Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302.



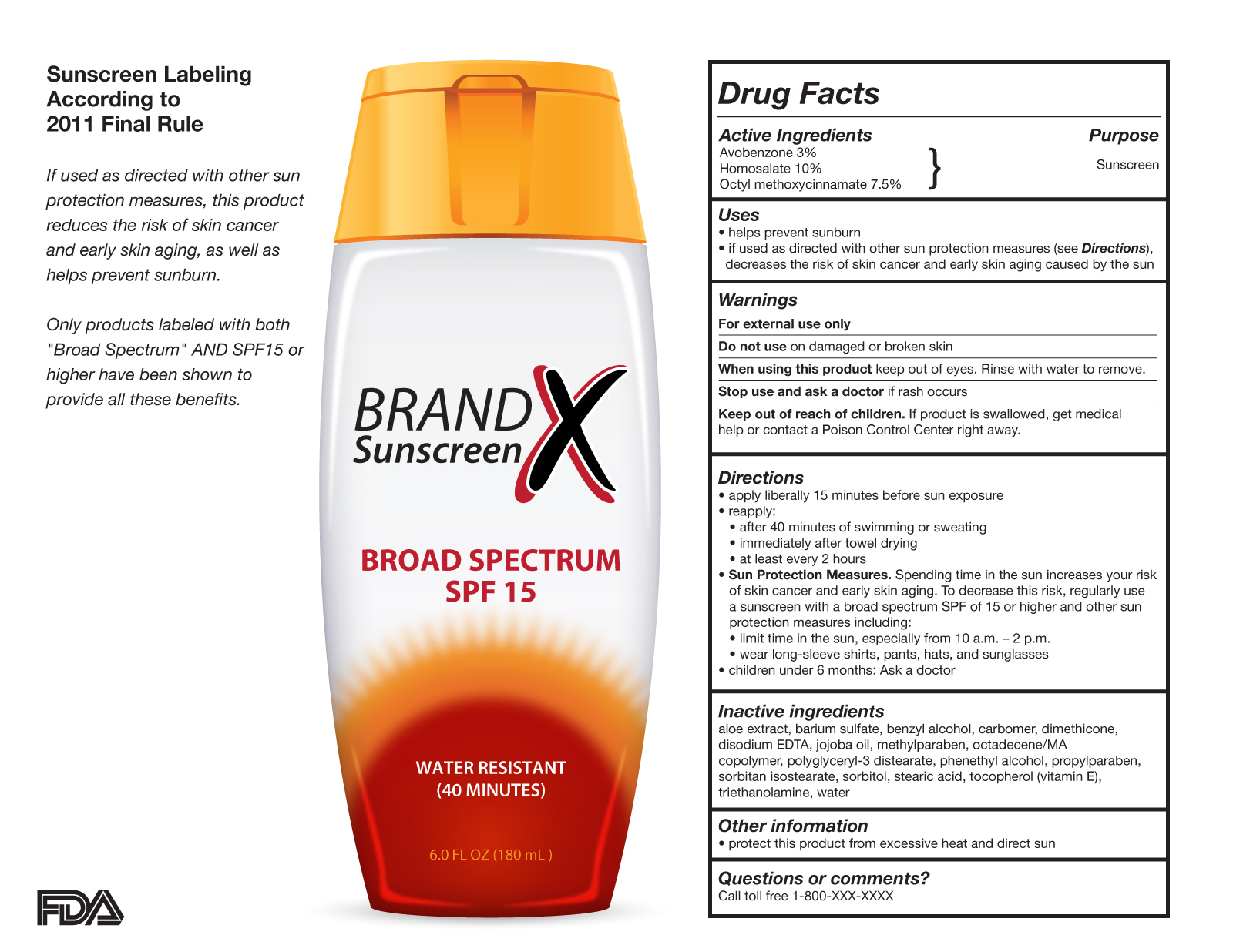
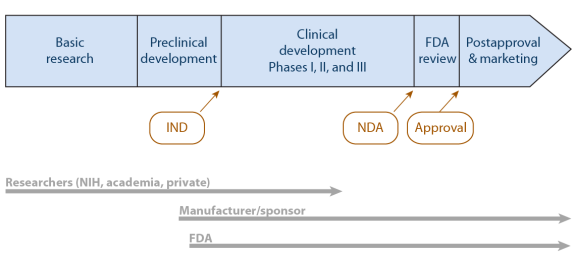

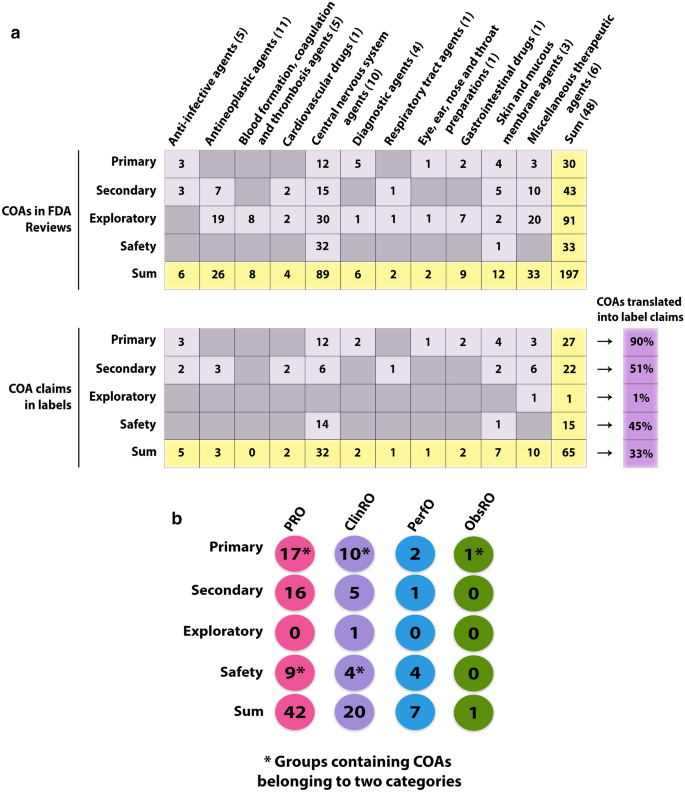

















Post a Comment for "45 fda structured product labels"